DERECHO TRIBUTARIO Y CONSTITUCIONAL DERECHO Y NUEVAS TECNOLOGIAS ACTUALIDAD JURIDICA Y ECONOMICA MEDIOAMBIENTE
Licencia Creative Commons

Esta obra está bajo una Licencia Creative Commons Atribución-NoComercial-SinDerivadas 4.0 Internacional.
Wednesday, April 26, 2023
Friday, April 21, 2023
JUDICIAL WATCH: LA FINANCIACIÓN POR EL NIH (USA) DE LA INVESTIGACIÓN DE CORONAVIRUS MUTADOS (20/04/2023)
This responds to your December 2, 2021, Freedom of Information Act (FOIA) request
addressed to the FOIA Office, National Institutes of Health (NIH). Department of Health
and Human Services’ (HHS) policy calls for the fullest possible disclosure provided by the
FOIA, 5 U.S.C. §552, consistent with the protections contained therein. The implementing
HHS Regulations establish the criteria pursuant to which the FOIA is administered, see 45
C.F.R. Part 5. Copies of the FOIA and the HHS FOIA Regulations are located at:
http://www.nih.gov/icd/od/foia/efoia.htm and http://www.nih.gov/icd/od/foia/cfr45.htm.
You requested All reports submitted by EcoHealth Alliance to NIH or its sub-agencies related to
NIH Grant No. 1R01AI110964 titled "Understanding the Risk of Bat Coronavirus Emergence"
during the term of the grant. (Date Range for Record Search: From 04/01/2014 To 12/02/2021).
NIH searched their files and found 552 pages of responsive records. The information being
withheld is protected from release pursuant to Exemptions 4 and 6 of the FOIA, 5 U.S.C. § 552
(b)(4) and (b)(6); and sections 5.31(d) and (f) of the HHS FOIA Regulations, 45 CFR Part 5.
Exemption 4 protects from disclosure trade secrets and commercial or financial information that
is privileged and confidential. Exemption 6 exempts from disclosure records the release of
which would cause a clearly unwarranted invasion of personal privacy.
You have the right to appeal this determination to deny you access to information in the
Agency’s possession. Should you wish to do so, your appeal must be sent within ninety (90) days
of the date of this letter, following the procedures outlined in Subpart F of the HHS FOIA
Regulations (https://www.federalregister.gov/documents/2016/10/28/2016-25684/freedom-ofinformation-regulations) to:
Revealed: NIH Funded Research at Wuhan Lab to Create Mutant Bat Coronaviruses and Study Their Capacity to Infect Humans
Records obtained by Judicial Watch show the National Institutes of Health funded an EcoHealth Alliance grant for sequencing the spike protein from coronaviruses obtained from bats to “creat[e] mutants to identify how significantly each would need to evolve to use ACE2,” which is explained as “the receptor to gain entry to human cells.”
Judicial Watch announced Wednesday it received 552 pages of records from the U.S. Department of Health and Human Services (HHS) that include the initial grant application and annual reports to the National Institutes of Health (NIH) from EcoHealth Alliance, describing the aim of its work with the Wuhan Institute of Virology (WIV) in China to create mutant viruses “to better predict the capacity of our CoVs [coronaviruses] to infect people.”
Eco Health Alliance planned to sequence the spike protein from coronaviruses obtained from bats for the purpose of “creating mutants to identify how significantly each would need to evolve to use ACE2,” which is explained as “the receptor to gain entry to human cells.”
Judicial Watch obtained the records through a Freedom of Information Act (FOIA) request it filed in December 2021 for:
“All reports submitted by EcoHealth Alliance to NIH or its sub-agencies related to NIH Grant No. 1R01A|110964 titled ‘Understanding the Risk of Bat Coronavirus Emergence” during the term of the grant.’”
In the initial “Application for Federal Assistance” submitted on June 5, 2013, by EcoHealth Alliance, a section is titled “Specific Aims,” which notes the intention to create mutant bat viruses and “predict the capacity of our CoVs [coronaviruses] to infect people”:
“To understand the risk of zoonotic CoV [coronavirus] emergence, we propose to examine 1) the transmission dynamics of bat-CoVs across the human-wildlife interface; and 2) how this process is affected by CoV evolutionary potential, and how it might force CoV evolution.
“We will assess the nature and frequency of contact among animals and people in two critical human-animal interfaces: live animal markets in China and people who are highly exposed to bats in rural China.”
“Specific Aim 3” discusses “Testing predictions of CoV inter-species transmission”:
“We will test our models of host range (i.e. emergence potential) experimentally using reverse genetics, pseudovirus and receptor binding assays, and virus infection experiments in cell culture and humanized mice.
“With bat-CoVs that we’ve isolated or sequenced, and using live virus or pseudovirus infection in cells of different origin or expressing different receptor molecules, we will assess potential for each isolated virus and those with receptor binding site sequence to spill over.
“We will do this by sequencing the spike (or other receptor binding/fusion) protein genes from all our bat-CoVs, creating mutants to identify how significantly each would need to evolve to use ACE2, CD26/DPP4 (MERS-CoV receptor) or other potential CoV receptors.”
In the continuing discussion of the aims of the research, the report states:
“In vitro [outside the body] cell lines & Humanized mouse model: We have developed primary cell lines and transformed cell lines from 9 bat species using kidney, spleen, heart, brain and intestine. We have used these for virus isolation, infection assays and receptor molecule gene cloning.
“We also have a large number of cell lines from humans and animals that we will use for virus infectivity assays. We have obtained a letter of support from Dr. Ralph Baric, who is keen to collaborate with us initially to infect his humanized mouse model with our bat SL-CoV [SARS-Like Coronavirus] that uses ACE2, and subsequently to use other CoVs that we identify …
“The results will provide information whether bat-CoVs could use known bat and human ACE2, DPP4 or other known CoV receptors to enter cells, and allow us to determine critical receptor binding sites, viral host range, and to better predict the capacity of our CoVs to infect people.”
EcoHealth Alliance’s $3.3 million grant to fund a project titled “Understanding the Risk of Coronavirus Emergence” was initially to run from Oct. 1, 2013, to Sept. 30, 2018. The first “Project/Performance Site Location” is the WIV.
Three other Chinese sites follow: East China Normal University in Shanghai, Yunnan Institute of Endemic Disease Control and Prevention in Dali and the Center for Disease Control and Prevention of Guangdong in Guangzhou.
On May 27, 2014, the NIH awarded EcoHealth Alliance $3,086,735 over five years for “Understanding the Risk of Bat Coronavirus Emergence.”
An EcoHealth Alliance grant application, received by the NIH on June 5, 2013, includes a list of “Senior/Key Personnel” including Shi Zhengli and Zhang Yun-Zhi of the WIV; Peter Daszak, CEO of EcoHealth Alliance; and other Chinese scientists, including Ke Changwen of the Chinese “CDC and Prevention of Guangdong Province.”
A section of the EcoHealth Alliance application titled “EcoHealth Alliance Budget Justification” describes some of the work to be conducted by EcoHealth scientists in China:
“A research scientist will be hired at 12 months time per year to provide direct assistance and oversight of field activities in China; maintain equipment and logistics; and coordinate animal and human sample shipment to the labs in China and in the US.
“Once we secure IRBs [Institutional Review Boards] for human sampling in Y1 [Year 1], we will hire three medical officers from China provincial CDCs [Centers for Disease Control] as consultants to work in Guangxi, Hunan, and Fujian during Y2-Y5. These medical officers will be responsible for IRB approved human sampling as well as maintaining cold chain for storage and shipping samples.
“Dr. Zhengli Shi, Senior Virologist. [Redacted] per year in Y1 -Y5. Dr. Shi will oversee the coronavirus screening for all samples collected in China. She will work with the PI [Principal Investigator], Co-Investigators, and Senior/Key Personnel to analyze data and write manuscripts. She will also coordinate data and material sharing with the co-investigators.”
In a budget calculation for the year 2014-15, the WIV as a sub-awardee of the grant was allocated to receive $128,718 in direct costs and $10,297 in indirect costs from NIH.
The salaries of Shi Zhengli and a WIV colleague Ge Xingyl are redacted from the budget. Over the five years of the grant, the Wuhan lab was to receive $749,976.
A section of the grant award titled “Wuhan Institute of Virology Budget Justification, Subaward” discusses “Other Direct Costs”:
- RNA extractions
We will be running RNA extractions for 1,000 bats per year (three samples per bat: oral, anal and blood) in each year … Extracted RNA per animal will be pooled.
- DNA Sequencing
In each year of the project, DNA sequencing will be performed on 3,200 samples at a cost of $2.91 per reaction. …
- Laboratory Supplies
We request support for in vitro infection experiments using pseudoviruses carrying the spike proteins (wild type or mutants) or live viruses in cell lines of different origins, binding affinity assays between the spike proteins (wild types or mutants) and different cellular receptor molecules and humanized mouse experiments.
The Year 2 annual report for the bat coronavirus project, budget period June 1, 2016, to May 31, 2017, under “Specific Aim 3,” states:
“Testing predictions of CoV inter-species transmission. The following experiments will be undertaken in Year 2:
-
- Humanized mice with human ACE2 receptors will be infected with WIV1 and the two rescued chimeric SARS-like coronaviruses to determine the tissue tropism and pathogenicity of bat SL-CoV.
- Isolation of novel bat coronaviruses. Live virus or pseudovirus will be used to infect cells of different origin or expressing different receptor molecules. Spillover potential for each isolated virus will be assessed.
- An infectious clone of full-length MERS-CoV [Middle East Respiratory Syndrome coronavirus] will be constructed using reverse genetic method. Using the S [spike] sequence of different MERS-related viruses identified from Chinese bats, the chimeric viruses with S gene of bat MERS-related coronaviruses and backbone of the infectious clone of MERS-CoV will be constructed to study the receptor usage and infectivity of bat MERS-related coronaviruses.
Among the “Additional Year 2 items for Specific Aim 3” are:
- The infectious clone of WIV1 was successfully constructed using reverse genetic methods;
- Two chimeric bat SARS-like coronavirus strains were constructed by replacing the S [spike] gene in the backbone of WIV1;
- Permission to import mice with human ACE2 to China was obtained, so as to conduct the experimental infections proposed in our R01 specific aims.
The annual report submitted for Year 3 of the grant project, budget period June 1, 2017, to May 31, 2018, under the heading “Specific Aim 3: Testing predictions of CoV inter-species transmission,” notes:
“In Year 3, we successfully isolated Rs4874 from the single [bat] fecal sample. Using the reverse genetic system we previously developed, we constructed two chimeric viruses with the WIV1 backbone replaced with the S [spike] gene of Rs7327 and Rs4231, respectively.
“Vero E6 cells were respectively infected with Rs4874, WIV1-Rs4231S and WIV1-Rs7327S, and efficient virus replication was detected by immunofluorescence assay in all infections.
“To assess the usage of human ACE2 by the three novel SL-CoVs, we conducted virus infectivity studies using HeLa cells with or without the expression of human ACE2. All viruses replicated efficiently in the human ACE2-expressing cells.”
In the Year 4 annual report, budget period June 1, 2018, to May 31, 2019, submitted to NIH by EcoHealth on Sept. 16, 2020, in answer to the question “How Have the Results Been Disseminated to Communities of Interest,” the report details that Peter Daszak and WIV lab director Shi Zhengli briefed their findings to, among others, the Defense Advanced Research Projects Agency, the National Natural Science Foundation of China, the Chinese Center for Disease Control and Prevention and the Chinese Academy of Sciences.
Among the accomplishments listed in the Year 4 report is:
“In vivo [experimentation done in a whole organism] infection of SARSr-CoVs with variants of S [spike] protein in human ACE2 (hACE2) expressing mice.”
The report also includes information about the construction of viruses of “varying pathogenicity” and testing them on humanized mice:
“Using the reverse genetic methods we previously developed, infectious clones with the WIV1 [bat SARS-like coronavirus] backbone and the spike protein of SHC014, W IV16 and Rs4231, respectively, were constructed and recombinant viruses were successfully rescued.
“In Year 4, we performed preliminary in vivo infection of SARSr-CoVs on transgenic mice that express hACE2. Mice were infected with 105 pfu of full-length recombinant virus of W IV1 (rWIV1) and the three chimeric viruses with different spikes.
“Pathogenesis of the 4 SARSr-CoVs was then determined in a 2-week course. Mice challenged with rWIV1-SHC014S have experienced about 20% body weight loss by the 6th day post infection, while rWIV1 and rWIV-4231 S produced less body weight loss.
“In the mice infected with rWIV1 -WIV16S, no body weight loss was observed (Fig. 35a). 2 and 4 days post infection, the viral load in lung tissues of mice challenged with rWIV1-SHC014S, rWIV1-WIV16S and rWIV1-Rs4231 S reached more than 106 genome copies/g and were significantly higher than that in rWIV1-infected mice (Fig. 35b). These results demonstrate varying pathogenicity of SARSr-CoVs with different spike proteins in humanized mice.”
In a revised award dated July 13, 2020, the NIH granted additional funds, including $77,750 to the University of North Carolina-Chapel Hill, $76,301 to the WIV and $75,600 to the Institute of Pathogen Biology of China.
The 2020 renewal application to extend funding for the Wuhan bat research projects states that EcoHealth would not be working with “select agents” (severe threats), such as SARS-CoV, but rather with a SARSr-CoV molecular clone designated WIV1 which, while a “BSL3” (biosafety level 3) pathogen, was not considered a select agent.
The select agent research was to be conducted at Baric’s lab at the University of North Carolina-Chapel Hill.
A section titled “P3CO Research” notes:
“Importantly, we are not proposing to genetically manipulate SARS-CoV over the course of this proposal. … However, we are proposing to genetically manipulate the full length bat SARSr-CoV WIV1 strain molecular clone during the course of this proposal, which is not a select agent, has not been shown to cause human infections, and has not been shown to be transmissible between humans.”
The same 2020 renewal application states:
“This project is a multi-institutional collaboration led by EcoHealth Alliance, New York (Daszak, PI), which will subcontract funds to three institutions: the Wuhan Institute of Virology (Dr. Shi), the University of North Carolina at Chapel Hill (Dr. Baric), and the Institute of Pathogen Biology (Dr. Ren).”
“A review of these and other documents strongly suggest that U.S. funding in China and elsewhere for mutant virus, gain-of-function research may have been responsible for the emergence of the COVID pandemic in Wuhan,” said Judicial Watch President Tom Fitton.
“This gain-of-function scandal should be the subject of criminal investigations.”
Through FOIA, Judicial Watch has uncovered a substantial amount of information about COVID-19 issues:
- HHS records included emails of then-Director of the NIH Francis Collins showing a British physicians’ group recommended the use of Ivermectin to prevent and treat COVID-19.
- Heavily redacted HHS records showed that just two days prior to FDA approval of the Pfizer-BioNTech COVID-19 vaccine a discussion was held between U.S. and U.K. health regulators regarding the COVID-19 shot and “anaphylaxis,” with the regulators emphasizing their “mutual confidentiality agreement.”
- Judicial Watch obtained HHS records regarding data Moderna submitted to the FDA on its mRNA COVID-19 vaccine, which indicated a “statistically significant” number of rats were born with skeletal deformations after their mothers were injected with the vaccine. The documents also revealed Moderna elected not to conduct a number of standard pharmacological studies on the laboratory test animals.
- Heavily redacted records from the FDA regarding the COVID-19 booster vaccine detailed pressure on COVID-19 booster use and approval.
- HHS records detailed internal discussions about myocarditis and the COVID-19 vaccine. Other documents detail adverse “events for which a contributory effect of the vaccine could not be excluded.”
- Judicial Watch uncovered HHS records detailing the extensive media plans for a Biden administration propaganda campaign to push the COVID-19 vaccine.
- HHS records revealed previously redacted locations of COVID-19 vaccine testing facilities in Shanghai, China. The FDA had claimed the name and location of the testing facilities were protected by the confidential commercial information exemption of the FOIA.
- NIH records showed an FBI “inquiry” into the NIH’s controversial bat coronavirus grant tied to the WIV. The records also show NIAID officials were concerned about “gain-of-function” research in China’s WIV in 2016. The Fauci agency was also concerned about EcoHealth Alliance’s lack of compliance with reporting rules and use of gain-of-function research in the NIH-funded research involving bat coronaviruses in Wuhan, China.
- Texas Public Information Act (PIA) records showed the former director of the Galveston National Laboratory at the University of Texas Medical Branch, Dr. James W. Le Duc, warned Chinese researchers at the WIV of potential investigations into the COVID-19 issue by Congress.
- HHS records regarding biodistribution studies and related data for the COVID-19 vaccines showed how a key component of the vaccines developed by Pfizer/BioNTech, lipid nanoparticles, were found outside the injection site, mainly the liver, adrenal glands, spleen and ovaries of test animals, eight to 48 hours after injection.
- Records obtained from HHS through a FOIA lawsuit related to hydroxychloroquine and COVID-19 revealed that a grant to EcoHealth Alliance was canceled because of press reports that a portion of the grant was given to the WIV.
- HHS records revealed that from 2014 to 2019, $826,277 was given to the WIV for bat coronavirus research by the NIAID.
- NIAID records showed that it gave nine China-related grants to EcoHealth Alliance to research coronavirus emergence in bats and was the NIH’s top issuer of grants to the Wuhan lab itself. The records also included an email from the vice director of the Wuhan Lab asking an NIH official for help finding disinfectants for the decontamination of airtight suits and indoor surfaces.
- HHS records included an “urgent for Dr. Fauci ” email chain, citing ties between the Wuhan lab and the taxpayer-funded EcoHealth Alliance. The government emails also reported that the foundation of U.S. billionaire Bill Gates worked closely with the Chinese government to pave the way for Chinese-produced medications to be sold outside China and help “raise China’s voice of governance by placing representatives from China on important international counsels as high level commitment from China.”
- HHS records included a grant application for research involving the coronavirus that appears to describe “gain-of-function” research involving RNA extractions from bats, experiments on viruses, attempts to develop a chimeric virus and efforts to genetically manipulate the full-length bat SARSr-CoV WIV1 strain molecular clone.
- HHS records showed the State Department and NIAID knew immediately in January 2020 that China was withholding COVID-19 data, which was hindering risk assessment and response by public health officials.
- HHS records show that NIH officials tailored confidentiality forms to China’s terms and that the World Health Organization conducted an unreleased, “strictly confidential” COVID-19 epidemiological analysis in January 2020.
- Fauci emails include his approval of a press release supportive of China’s response to the 2019 novel coronavirus.
Take a look at page 127:
The above page describes “receptor mutants and pseudoviruses,” specifically a mutant of HIV backbone with SARS-like “spike proteins.” These NIH-funded researchers created these HIV-SARS mutants to see how well they would infect human lungs and experimented on “humanized mice” whose lung cells resembled human cells.
These mutant viruses are, in a way, the opposite of Sars-Cov-2. They represent an HIV backbone with SARS-like spike protein.
Sars-Cov-2, on the contrary, is a coronavirus backbone with spike-protein carrying HIV genes. (Such a chimera is described in the DEFUSE proposal submitted by Peter Daszak to another government agency)
Airborne HIV?
HIV is an exceptionally cunning virus: it integrates into the human DNA after infection. Such integration is called “reverse transcription,” whereby RNA from the virus becomes part of the DNA of human cells. HIV essentially edits the human genome and inserts its code into it. After that, human cells produce new HIV viral particles, as this NIH page explains.
However, HIV is not very contagious. It cannot target cellular receptors found in human lungs, for example. One cannot get HIV via the airborne route: HIV infections require blood-to-blood transmission, and HIV-carrying aerosols cannot infect human respiratory systems.
A pseudovirus that can infect human ACE2 cells, which are present in our lungs, is one step closer to airborne transmission: an aerosol carrying ACE-2-infecting HIV mutant could infect someone upon being breathed in.
Is the research that creates ACE-2-infecting HIV mutants, which may be contagious via the respiratory route, safe? What if these recombinants escape the laboratory?
And yet, in search of grant money, fame, and discoveries, virologists funded by the NIH conducted such experiments involving ACE2-infecting HIV chimeras with minimal oversight.
Their work gave us Sars-CoV-2 and the Covid pandemic. Again, Sars-Cov-2, a recombinant chimera carrying HIV genes on a coronavirus backbone, is NOT the same as the pseudovirus described above; it is something else but related.
The above shows that work on HIV/SARS coronavirus recombinants, funded by the NIH, was conducted by the same people whose cooperation with the Wuhan Institute of Virology gave us the Covid pandemic.
Almost every human was infected with the HIV-gene-carrying COVID virus. Every COVID-vaccinated human was injected with spike-protein-producing mRNA, which encoded the same HIV genes.
Fact-Checkers Tried to Distract Us
Numerous fact-checking articles attempted to deny the link between HIV and Sars-Cov-2.
 |
Those assurances are false.
The document I highlighted shows evidence that the same people who gave us Sars-Cov-2 also worked on HIV/SARS chimeras. Therefore, there is a “plausible route” by which the Covid pandemic virus could carry HIV genes. This belies the fact-checkers’ hollow claims.
Casi todos los humanos han sido infectados con el #virus #COVID que porta #genes del #virus del #SIDA. Casi todos los humanos han sido vacunados con la "spike protein" que produce #mRNA que codifica los mismos #genes del #virus del #SIDA
— Guillermo Ruiz Zapatero (@ruiz_zapatero) April 23, 2023
ASÍ CONSTA EN LOS DOCUMENTOS FOIA https://t.co/Wbd54UZWLu
Where is CTCCTCGGCGGGCACGTAG in the Moderna Patent
Igor Chudov
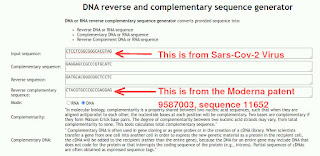
Two astute readers asked me a very reasonable question: where in the Moderna patent 9,587,003, exactly, is something that matches the Sars-Cov-2 sequence CTCCTCGGCGGGCACGTAG? I am trying to explain here. We get: CTACGTGCCCGCCGAGGAG in the Moderna patent sequence 11652.
Go to the “Reverse Complement Calculator”: https://bugaco.com/calculators/dna_reverse_complement.php
Now type in the genes from Sars-Cov-2 (which is CTCCTCGGCGGGCACGTAG):
So, this shows that the Sars-Cov-2 sequence CTCCTCGGCGGGCACGTAG, matches the Moderna patent sequence CTACGTGCCCGCCGAGGAG as a “reverse complement”.
This is the explanation of “reverse complement”.
Remember that Sars-Cov-2 is a RNA virus and Moderna patents are DNA
(DNA STRUCTURE AND THE REVERSE COMPLEMENT OPERATION)
None of this tells us whether this sequence is “good”, “bad”, “pure chance”, etc.
All we know is: CTCCTCGGCGGGCACGTAG is the most crucial part of Sars-Cov-2 RNA, containing the code for the “furin cleavage site”
No natural coronaviruses contain a “furin cleavage site”
Furin cleavage site greatly enhances infectivity of Sars-Cov-2 and its ability to infect humans and other species
This code only appears in a Moderna oncology (cancer) patent 9587003, and not in any multicellular organisms or viruses known before 2020
The mutation of MSH3 gene related to this patent, is known to break DNA recombination and lead to cancers (see my previous article)
Spike protein is also known to penetrate cell nuclei and interfere with V(D)J DNA recombination and BRCA1 and p53 repair, for a reason that MAY OR MAY NOT be related to CTCCTCGGCGGGCACGTAG
BRCA mutation is responsible for breast and ovarian cancer
https://thoreaucastellano.blogspot.com/2022/02/where-is-ctcctcggcgggcacgtag-in-moderna_24.html
Thursday, April 20, 2023
AUTO TRIBUNAL SUPREMO 13-04-2023 REC. CASACIÓN 3498/2022 ( BASE SANCIONES TRIBUTARIAS EN OPERACIONES ENTRE SOCIEDADES VINCULADAS (II))
#AUTO #TRIBUNALSUPREMO 13-04-2023 REC. CASACIÓN 3498/2022 ( #BASE #SANCIONESTRIBUTARIAS EN OPERACIONES ENTRE #SOCIEDADES #VINCULADAS) https://t.co/2nTny9TJT5
— Guillermo Ruiz Zapatero (@ruiz_zapatero) April 20, 2023
(1)
Determinar cuál debe ser la base de cálculo de la sanción tributaria
(2)
— Guillermo Ruiz Zapatero (@ruiz_zapatero) April 20, 2023
en aquellos supuestos de regularización de operaciones vinculadas en los que se imputan a una entidad rentas que a su vez fueron declaradas por otras sociedades y dieron lugar a devoluciones en las regularizaciones practicadas a estas https://t.co/b1vHoh9foq
(3)
— Guillermo Ruiz Zapatero (@ruiz_zapatero) April 20, 2023
determinando si aquella debe ser, bien la cantidad dejada de ingresar por la entidad inspeccionada o, por el contrario, la diferencia entre esta cantidad y la cantidad ingresada por la sociedad vinculada respecto de las mismas rentas cuya devolución se reconoce https://t.co/VYH0WXuhOx
AUTO TRIBUNAL SUPREMO 13-04-2023 REC. CASACIÓN 3498/2022 ( BASE SANCIONES TRIBUTARIAS EN OPERACIONES ENTRE SOCIEDADES VINCULADAS)
SÉPTIMO.- Cuestión en la que se encuentra un interés casacional objetivo.
La cuestión en la que esta Sala considera que puede hallarse un interés casacional suficientemente caracterizado es la siguiente:
Determinar, a la luz de los principios de proporcionalidad, íntegra regularización y buena administración, cuál debe ser la base de cálculo de la sanción tributaria prevista en el artículo 191 de la LGT en aquellos supuestos de regularización de operaciones vinculadas en los que se imputan a una entidad rentas que a su vez fueron declaradas por otras sociedades y dieron lugar a devoluciones en las regularizaciones practicadas a estas, determinando si aquella debe ser, bien la cantidad dejada de ingresar por la entidad inspeccionada o, por el contrario, la diferencia entre esta cantidad y la cantidad ingresada por la sociedad vinculada respecto de las mismas rentas cuya devolución se reconoce por la administración tributaria.
Las demás cuestiones propuestas no guardan real relevancia con la decisión impugnada si se atiende a la ratio decidendi de la sentencia recurrida, puesto que la desestimación del motivo relativo a la posible deducción como gasto en el IS de unas cuotas de IVA soportadas se basó en última instancia en la imposibilidad de admisión de la deducibilidad del gasto consistente en el pago de unas cantidades facturadas a la recurrente por las sociedades vinculadas, siendo reseñable en este punto además que la cuestión de la regularización íntegra fue desestimada con fundamento en un desistimiento manifestado por la actora en sede económico-administrativa y en la interpretación que de su alcance se hizo en la instancia.
OCTAVO. - Justificación suficiente de que el recurso planteado cuenta con interés casacional objetivo para la formación de la jurisprudencia.
Debe reseñarse que, aunque en un principio esta Sección optó por inadmitir el recurso por encontrarse vinculada por la decisión tomada en un recurso coincidente con el presente, en concreto por la providencia de inadmisión dictada en el recurso de casación n.º 1505/2022, frente a la que no se formuló incidente de nulidad, la coincidencia entre la controversia latente en los recursos 8550/2021, admitido por auto de 22 de junio de 2022, y 2453/2022, admitido por auto de 20 de octubre de 2022, y la que acaece aquí, hace obligado reexaminar aquella decisión y reconocer la existencia de las razones que condujeron a la admisión de estos últimos.
En este punto, conviene señalar que la sentencia recurrida, con remisión a otras que han sido citadas líneas arriba, desestimó la alegación relativa a la inexistencia de perjuicio económico por causa de la práctica de un ajuste bilateral efectuado en la sociedad recurrente y en las vinculadas, lo que trató de acreditarse por la actora mediante una prueba pericial. Esta desestimación se basó en que esta consideración se extrajo de un análisis ex post de la situación tributaria de la recurrente y de las sociedades vinculadas, no anterior a la regularización practicada. Aunque este razonamiento se incluye en el fundamento dedicado al motivo relativo a la ausencia de ventaja tributaria derivada de las facturas simuladas emitidas por las sociedades vinculadas, es evidente que la Sala a quo, al confirmar la decisión administrativa en todos sus aspectos y al hacer lo propio con la sanción, prescindió de las consecuencias que pudiera haber comportado este ajuste bilateral en la imposición de la sanción.
Por lo expuesto, y como se concluyera en los antedichos autos de admisión, ha de admitirse el recurso de casación por apreciarse la existencia de la circunstancia descrita en el apartado c) del art. 88.2 de la LJCA, al trascender la cuestión del caso concreto, así como por darse la presunción del apartado a) del artículo 88.3 de la LJCA, por inexistencia de jurisprudencia concluyente sobre la forma de determinación de la base de la sanción en los casos en que aunque se haya producido una falta de ingreso se haya practicado un ajuste bilateral en el que resultan implicadas partes vinculadas que ha determinado un perjuicio a la Hacienda Pública menor que el que se deriva a priori de esa falta de ingreso.
Finalmente, como se hiciera en el auto de 22 de junio de 2022, debe traerse a colación el principio de buena administración que, merced a lo establecido en el artículo 41 de la Carta de los Derechos Fundamentales de la Unión Europea, ha adquirido el rango de derecho fundamental en el ámbito de la Unión, calificándose por algún sector doctrinal como uno de los derechos fundamentales de nueva generación del que se ha hecho eco la jurisprudencia de este Tribunal Supremo desde la sentencia de 30 de abril de 2012, dictada en el recurso de casación 1869/2012 (ECLI:ES:TS:2012:3243), pasando por la sentencia, con abundante cita, 1558/2020, de 19 de noviembre, dictada en el recurso de casación 4911/2018 (ECLI:ES:TS:2020:3880), que se ha querido vincular, en nuestro Derecho interno, a la exigencia que impone el artículo 9.3º de nuestra Constitución sobre la proscripción de la arbitrariedad en la actuación de los podres públicos, pero que, sobre todo, debe considerarse implícito en la exigencia que impone a la actuación de la Administración el artículo 103, en cuanto al sometimiento «pleno» a la ley y al Derecho. Y en ese sentido, es apreciable la inspiración de la exigencia comunitaria en el contenido de los artículos 13 y 53 de la Ley del Procedimiento Administrativo Común de las Administraciones Públicas, al referirse a los derechos de los ciudadanos en sus relaciones con la Administración.
En este sentido, nuestra sentencia de 3 de diciembre de 2020 (RCA/8332/2019: ECLI:ES:TS:2020:4161) señala que «(l)a buena administración es algo más que un derecho fundamental de los ciudadanos, siendo ello lo más relevante; porque su efectividad comporta una indudable carga obligación para los órganos administrativos a los que se les impone la necesidad de someterse a las más exquisitas exigencias legales en sus decisiones, también en las de procedimiento.» (Sic).
Conviene, por lo tanto, un pronunciamiento del Tribunal Supremo que, cumpliendo su función uniformadora, sirva para dar respuesta a la cuestión nuclear que suscita este recurso de casación a fin de dotar de un criterio unívoco a la administración y a los tribunales de justicia españoles.
NOVENO. - Admisión del recurso de casación. Normas que en principio serán objeto de interpretación.
1. En virtud de lo dispuesto en el artículo 88.1 de la LJCA, en relación con el artículo 90.4 de la LJCA, procede admitir este recurso de casación, cuyo objeto será, por presentar interés casacional objetivo para la formación de la jurisprudencia, la cuestión descrita en el razonamiento jurídico séptimo.
2.
2. Las normas que, en principio, serán objeto de interpretación son pues los artículos 3.2, 178 y 191.1 de la Ley 58/2003, de 17 de diciembre, General Tributaria; 131.3 de la Ley 30/1992, de 26 de noviembre, de Régimen Jurídico de las Administraciones Públicas y del Procedimiento Administrativo Común, y 29.3 de la Ley 40/2015, de 1 de octubre, de Régimen Jurídico del Sector Público; 41 de la Carta de los Derechos Fundamentales de la Unión Europea, y 9.3 y 103 de la Constitución Española.
Ello sin perjuicio de que la sentencia haya de extenderse a otras si así lo exigiere el debate finalmente trabado en el recurso, ex artículo 90.4 de la LJCA.
Wednesday, April 19, 2023
EVIDENCIAS DE SERIAS DEFICIENCIAS EN LA SUPERVISIÓN POR LAS AUTORIDADES SANITARIAS DE LAS VACUNAS COVID (UK)
Hard hitting report which calls for immediate suspension of the mRNA ‘vaccines’ and a full investigation into how the MHRA authorised them in the first place. Both of which I have been calling for in Parliament since December. pic.twitter.com/m4UHjhIxfA
— Andrew Bridgen (@ABridgen) April 19, 2023